RML Quality Assurance Program
“Continuous Efforts and Execution lead to quality excellence”
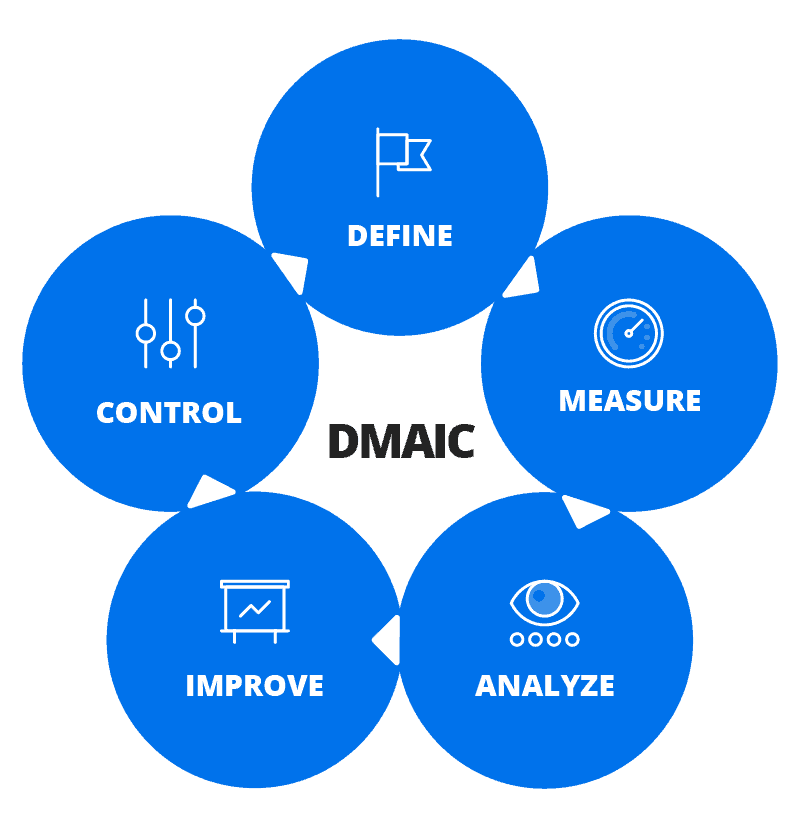
The term RML-Quality Assurance Program (RML-QAP) is seen as a powerful tool to help laboratories to demonstrate their competence in sample testing. QAP enables laboratories to monitor their accuracy and quality of results over time, identify trends and help them to take necessary corrective action.